Computational Modeling and Simulation (CM&S), aka in silico methods, is establishing itself as a powerful tool to support marketing applications of a broad range of medical devices.
Regulators are increasingly open to accepting computer-generated evidence from modeling disciplines like solid mechanics, fluid dynamics, electromagnetics, and heat transfer analysis to support the evaluation of medical devices.
CM&S comes with the promise of an additional source of evidence by filling gaps in clinical data, increasing the statistical power of the studies, and providing a better understanding of the device’s behavior. In silico methods bring additional evidence that can increase confidence in the expected performance of the device, and reduce the number of revision rounds during conformity assessment.
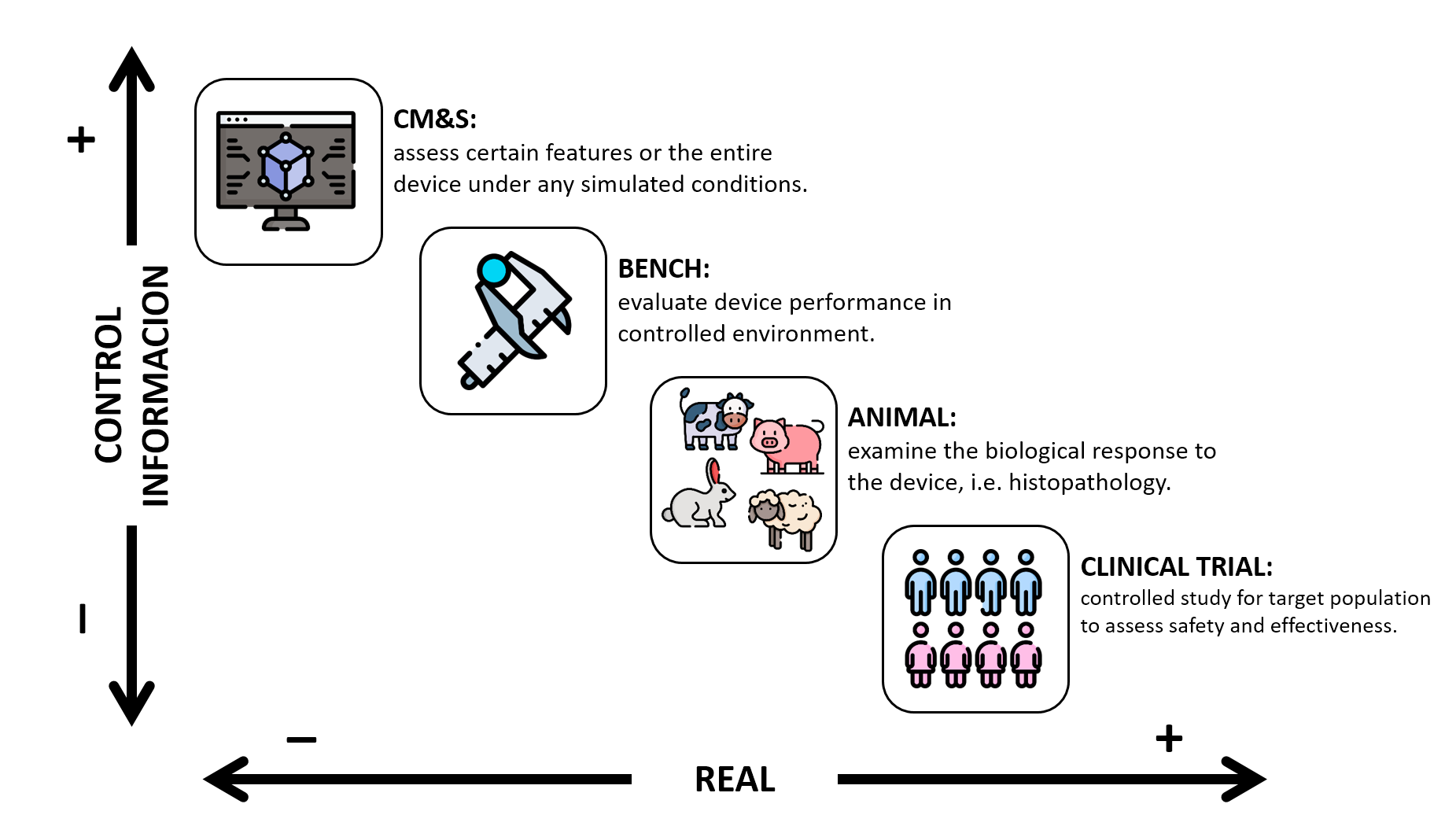
You can imagine CM&S as an additional tool in the toolkit to provide evidence on the safety and effectiveness of a medical device where each type of model fits within the information-control-vs-reality spectrum.
The FDA has identified several ways that CM&S can potentially be used to support a regulatory submission, including but not limited to:
- In Silico Device Testing: Computational models used to generate information supporting device safety and/or effectiveness.
- CM&S used within medical device software: Computational models implemented as device software functions.
- In Silico Clinical Trials: Computational models where the device performance is evaluated using a ‘virtual cohort’ of simulated patients.
- CM&S-based qualified tools: Computational models used to develop or evaluate medical devices.
Acceptance of CM&S studies (in silico evidence) by regulatory bodies
United States of America

Within the in silico adoption, FDA is helping break ground. In 2011, FDA developed a strategic plan for regulatory science which identified an important role for CM&S in its strategic priorities. Since then, FDA has undertaken several initiatives to add clarity to the regulatory pathway.
In 2014, FDA released the draft of the guidance on Reporting Computational Modeling Studies in Medical Device Submissions. Finally issued in 2016, this document became the first guideline for in silico evaluation for medical device submission worldwide. This document guides on what the FDA staff expects from sponsors when reporting CM&S studies.
In 2017, the results of an FDA-initiated multi-laboratory study to create well-defined benchmark flow models were published. The study entitled, FDA Benchmark Medical Device Flow Models for CFD Validation, was intended to establish reliable and publicly available experimental datasets to validate CFD models used to develop blood-contacting medical devices.
During this period, FDA was also working alongside medical device industry partners through the Medical Device Innovation Consortium in a framework to establish model credibility for computational models used in the evaluation of medical devices. These efforts were materialized in the ASME standard V&V 40-2018 Assessing Credibility of Computational Modeling Through Verification and Validation: Application to Medical Devices.
This standard was rapidly adopted by FDA, which by the end of 2021 published the Draft Guidance for Assessing the Credibility of Computational Modeling and Simulation in Medical Device Submissions. This document outlines the V&V40 principles in a step-by-step manner and opens the door to a broader range of real-world datasets for validation.
Just a few weeks ago, FDA published the first report on modeling and simulation, entitled Successes and Opportunities in Modeling & Simulation for FDA. The report showcases 14 success stories on how FDA scientists use CM&S approaches for scientific research and regulatory decision-making. This is one more step of the FDA towards the establishment of CM&S as a standard tool for regulatory decision-making.
All in all, the FDA is not only embracing but encouraging the medical device industry to adopt in silico methodologies to improve the assessment of medical device performance.
European Union

The use of computationally generated evidence in the European Union is less clear than in the US. It is true that the new Medical Device Regulation explicitly acknowledges the use of CM&S, for example in annex VII, 4.5.4.a states “the pre-clinical testing, for example, laboratory testing, simulated use testing, computer modelling, the use of animal models,”. However, with regard to the validation required for in silico studies, it only states that it must be demonstrated beforehand.
Within the European Union, there are several initiatives to promote the adoption of in silico methods. The most prominent actor in this space is the Avicenna Alliance, an association of industry, academia, and healthcare organizations, which developed a “Roadmap for in silico medicine” back in 2016. The Alliance now seeks to put this roadmap into policy and ensure the development of a well-functioning framework for the in silico medicine ecosystem.
Given the importance of notified bodies for CE marking of medical devices, Avicenna Alliance published in 2021 the Notified Bodies Landscape and Status. The whitepaper maps the perception of in silico evidence among notified bodies. The document identifies as one of the main challenges the lack of guidance from regulators, to better understand what they can accept and what not in terms of in silico testing.
Another initiative ongoing since 2020 from the Avicenna Alliance is the Community Challenge towards Consensus on Characterization of Biological tissue. The challenge aims to achieve community consensus regarding the testing protocols for material characterization of biological tissues, in a way that can reduce the variability and increase the reproducibility of tissue properties used in CM&S studies.
More recently, in 2022 the position paper Toward a Regulatory Pathway for the Use of In Silico Trials in the CE Marking of Medical Devices was published. The paper proposes a possible trajectory for wider adoption of In Silico Trials solutions in the CE Marking system for medical devices.
Finally, another ongoing initiative that will soon bear fruit is the project EU-STANDS4PM – A European standardization framework for data integration and data-driven in silico models. The project is initially intended for personalized medicine applications but the standardization framework will also be applicable to CM&S in the medical device sector. This initiative is the germ of an ISO standard on predictive models for in silico medicine.
In conclusion, the use of CM&S in the regulatory submission of medical devices in the European Union today is unclear. However, there are many ongoing initiatives that will pave the way in the coming years toward the adoption of in silico methods in the CE marking of medical devices.
Japan

The Japanese Ministry of Economy, Trade, and Industry in collaboration with the Ministry of Health, Labour, and Welfare, issues official guidelines for Medical Device Development elaborated in cooperation with industry and academia. Up until today, there are 52 publicly available handbooks dated from 2007.
Guidance 39 “Development guidelines for in silico evaluation”, issued in march 2019, aims to illustrate the basic concepts of in silico assessment for the development of medical devices, particularly during the technical assessment phase. The devices targeted in this guideline are those that are expected to be difficult to evaluate in vitro (bench) or in vivo due to ethical, time, or cost considerations.
The guidance points out that in silico evidence open the possibility to shorten the development time/cost, avoid ethical problems, and collect large quantities of high-quality information which cannot be obtained by experiments alone. Some examples of expected impact are:
- Streamline evaluation methods that require long evaluation periods and high costs, such as clinical trials, in order to speed up development.
- Alternative methods when clinical trials cannot be conducted rationally for ethical, economic, statistical, or other reasons, or when in silico evaluations can be conducted more rationally.
- Alternative method when it is unclear whether the results of animal experiments can be applied to humans (extrapolation) or when validation of the extrapolation is time-consuming and costly.
- Determine the experimental conditions for in vitro and in vivo evaluations, especially the upper and lower limits, possible values, and the number of samples.
- Evaluate the efficacy and safety of medical devices for each individual patient.
- Predict unknown phenomena (unknown hazards, etc.) that may occur in a subject.
In the supplementary material, the guidance cites the V&V guidelines from ASME, the CM&S results reporting guidelines from the FDA, and early initiatives of Avicenna Alliance to promote in silico methodologies in Europe.
Furthermore, from the National Institute of Technology and Evaluation of Japan (NITE) there is a publicly available slide deck of training material for the use of in silico evaluation methods from 2020.
In light of this, the Japanese market of medical devices shows already steps toward the adoption of CM&S results for the technical evaluation of medical devices and is well aligned with US and EU initiatives.
United Kingdom

On 22 march 2022, Innovate UK KTN launched a new UK-wide network called InSilicoUK. A community driving innovation and growth for patient safety and benefit using in silico evidence and regulatory science. During the launch event, industry leaders highlighted the crucial importance of regulatory science and innovation for the UK economy, its international dimension, and the key role of standards and regulators.
InsilicoUK point towards the same benefits of adopting in silico clinical trials as the Japanese guidelines. In particular, they showcase a real example of how an in silico trial of intracranial flow diverters expands insights from conventional clinical trials.
So far, InsilicoUK has run a couple of community surveys regarding in silico adoption. Survey #1 “Enablers and Barriers to adoption of in silico methods as a source of regulatory evidence” identified the main barriers:
- the uncertainty on the regulatory acceptance by the regulatory bodies.
- the scientific maturity and model credibility of CM&S studies.
And the main enablers
- the development of international standards and best practices.
- a network that supports in silico trials.
Finally, regulators and policymakers were identified as the first stakeholders to reach for driving the adoption of in silico trials. All the details of the survey results are accessible from the InSilicoUK slack channel.
Meanwhile, survey #2 “In Silico Trials: Market Size & Areas of Early Adoption in Medical Product Innovation & Regulation” is still open and you can participate here.
Other initiatives like the Roadmap for the Software and AI as a Medical Device Change Programme recently updated by the Medicines and Healthcare products Regulatory Agency of the UK (MHRA) will help to add regulatory clarity for the use of CM&S in the medical device industry.
With these initiatives, the UK joins the group of innovative regulatory agencies that is looking into the adoption of CM&S as a tool to improve the assessment of medical devices.
The future of CM&S in the medical device industry
Computer Modeling and Simulation have revolutionized the automotive and aerospace industries, minimizing the number of physical tests. Today, the norm is to perform all design interactions and performance optimization in the virtual environment dramatically reducing cost, accelerating design, and improving the safety of new models coming to market.
However, the medical device industry still relies heavily on lengthy and costly clinical trials to develop new products, which limits companies’ ability to develop innovative products and leaves new/smaller companies with high market entry costs to bring new products to market.
In silico methodologies will play a growing and fundamental role in the development and de-risking of new medical devices in the future. For example, they will offer the opportunity to address questions that cannot be addressed clinically due to financial or ethical considerations. They will also facilitate the exploration of using a medical device in populations that cannot be investigated clinically, such as in patients with rare diseases or pediatric patients, without harm.
Although the regulatory bodies work at the national level, there are growing efforts in the CM&S community to develop guidelines involving as many stakeholders as possible to achieve global consensus that clears the path for the adoption of in silico methodologies in the medical device and pharma industries.
The initiatives presented here give a good idea of the trend in the medical device industry toward adopting in silico methods to evaluate the safety and effectiveness of medical devices and accelerate the time to market new products.
Companies that do not embrace this technology could be left behind.
